News
Posted: November 28, 2022 | News, Press
Amsino Announces US FDA 510(K) Clearance for the AMSafe® NeuFlo™ Needleless Connector
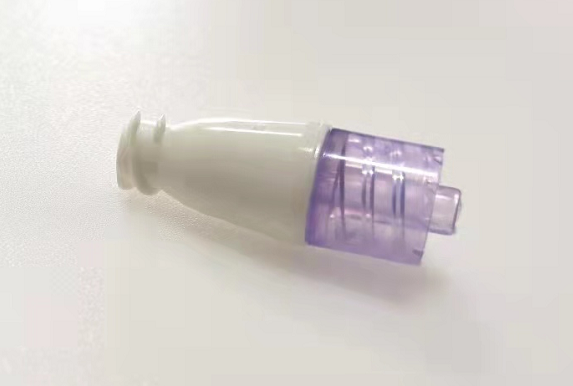
Pomona, California, November 28, 2022- Amsino Medical Group Company is pleased to announce that it has received the U.S. Food and Drug Administration’s 510(k) clearance for its AMSafe® NeuFlo™ Needleless Connector.
AMSafe® NeuFlo™ Needleless Connector is a single use, sterile, non-pyrogenic device intended for needleless access to intravascular administration set for the administration of fluids to a patient through a cannula placed in the vein or artery.
As the latest addition to the AMSafe® Medication Delivery Systems product line, the NeuFlo™ Needleless Connector provides infection control technology that is recognized throughout the industry to help reduce bacterial contamination. The new NeuFlo™ Needleless Connector is an ideal complement to the comprehensive AMSafe® product portfolio, including the negative pressure Sure-Lok®, that prioritizes safety first.
Amsino President and CEO, Dr. Richard Y. Lee, shares “This 510(k) approval came timely as we are prepared to further enhance and expand our IV portfolio as a strategic growth driver for the company in 2023 and beyond.”
Amsino is dedicated to improving and strengthening health and well-being around the world. The new AMSafe® NeuFlo™ Needleless Connector is an additional advancement of Amsino’s commitment to improve safety in the healthcare industry worldwide.