News

Posted: November 25, 2024 | News, Press
Amsino Expands North American Manufacturing and Doubles Capacity with the Acquisition of MedXL Inc.
Amsino International Inc. announced the successful closing of transactions today to acquire the assets of MedXL, Inc., located in Quebec, Canada. This acquisition includes all existing assets and infrastructure at MedXL facilities, such as intellectual properties, real estate properties, manufacturing equipment, and global product registrations in approximately 60 countries.

Posted: June 26, 2024 | News, Press
Amsino Medical Group Company announces transformational investment from Novo Tellus
Amsino Medical Group, a global provider of medical consumable devices, is pleased to announce the closing of a substantial investment by Novo Tellus, a leading investment firm specialising in long-term investments in the global supply chain.

Posted: May 11, 2023 | News, Press
Amsino International to Exhibit at China International Medical Equipment Fair (CMEF)
Amsino International is excited to be exhibiting at the China International Medical Equipment Fair (CMEF) May 14-19, 2023. The event will be held at the National Exhibition and Convention Center in Shanghai, China. Be sure to visit Amsino in the International Hall (Hall 6, Floor 2) at Booth #D24. Our team looks forward to the opportunity to meet and network with our fellow industry leaders throughout the event.

Posted: March 21, 2023 | News, Press
Amsino Awarded Suction and Fluid Management Product Agreement with Premier, Inc.
Amsino has been awarded a US national group purchasing agreement for Suction and Fluid Management products including Suction Canisters, Suction Liners, Yankauers, and Suction Tubing with Premier, Inc. Effective 1 May 2023, the new agreement allows Premier members, at their discretion, to take advantage of special pricing and terms pre-negotiated by Premier for Suction and Fluid Management products.

Posted: March 17, 2023 | News, Press
Amsino Awarded “Most Valuable Partner” Award from Bound Tree
On Monday, February 27, 2023, Amsino Medical Group was honored to be awarded the “Most Valuable Partner” award from Bound Tree. Our team attended the Bound Tree National Sales Meeting on Monday, February 27th in Orlando, FL where Beth Clifford, Phil Kelsey, and Lowell Warner graciously accepted the award.
Amsino was awarded for our partnership with Bound Tree throughout 2022. We were able to supply critical market needs for globally scarce Pre-Filled Saline Flush Syringes.
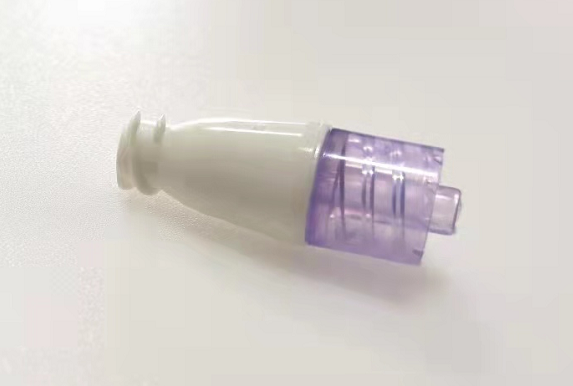
Posted: November 28, 2022 | News, Press
Amsino Announces US FDA 510(K) Clearance for the AMSafe® NeuFlo™ Needleless Connector
Pomona, California, November 28, 2022- Amsino Medical Group Company is pleased to announce that it has received the U.S. Food and Drug Administration’s 510(k) clearance for its AMSafe® NeuFlo™ Needleless Connector.
AMSafe® NeuFlo™ Needleless Connector is a single use, sterile, non-pyrogenic device intended for needleless access to intravascular administration set for the administration of fluids to a patient through a cannula placed in the vein or artery.
Posted: April 1, 2021 | Press
RECEPTAL TWIST: A genuine closed system for suction and fluid management in a healthcare facility
Pomona, California - Amsino International is committed to deliever the most innovative and safe technology for your suction and fluid management needs. RECEPTAL Twist introduction to the market is one more tep for Amsino in the pursuit of patient and caregiver safety.
Posted: March 1, 2021 | Press
Safety When Clearing the Airway
When a patient is in respiratory failure and requires ventilatory support an Endotracheal Tube (ETT) is inserted by an expert physician or respiratory therapist in the trachea and placed on a ventilator to support breathing. If a patient requires prolonged mechanical ventilation support a tracheostomy tube (Trach) is surgically inserted. In both cases, the upper airway is bypassed and function of the nose to filter, warm and humidify the air is no longer present.
Posted: February 18, 2021 | Press
Amsino Announces US FDA 510(k) Clearance for the Puggle® Enteral Feeding Pump and Set
Pomona, California, February 18, 2021- Amsino Medical Group is pleased to announce that it has received the U.S. Food and Drug Administration’s 510(k) clearance for its Puggle® Enteral Feeding Pump and Set.